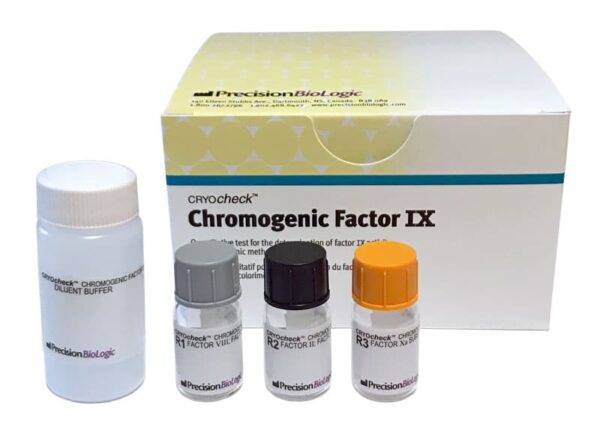
Chromogenic Factor IX
Diagnostic KitsCRYOcheck™ Chromogenic Factor IX is a chromogenic assay for the determination of factor IX activity in human plasma. It is intended to be used as an aid in the management of hemophilia B in individuals aged two years or older. With a limit of quantification of 0.5% FIX activity and a test range of 0–200% FIX using one standard curve*, CRYOcheck Chromogenic Factor IX provides unsurpassed assurance in results, especially in cases of patients with severe hemophilia B.
With the ability to store thawed reagents at 2 to 8°C for up to 48 hours, or refreeze for up to one month, labs of all sizes can meet their testing requirements while minimizing wastage.
- Convenient frozen format ready to use within minutes, no reconstitution errors
- Intended for use on automated coagulation analyzers
- Only one calibration curve used for entire measuring range of 0–200%*
- 24-hour on-board stability
- Excellent precision at low FIX activity
* may vary based on the instrumentation in use
Specifications
Storage and Shelf Life
Storing
below -70°C
Type
Frozen
Expiration
3 years after manufacturing date
Available Formats
| Cat # | Format |
|---|---|
| CCCF09 |
4 x 0.75 mL (Reagent 1) 4 x 2.3 mL (Reagent 2) 4 x 1.0 mL (Reagent 3) 4 x 10.0 mL (Diluent Buffer) |
Webinar
Issues in Factor IX Activity TestingLearn about current issues in FIX activity testing with Julie Tange, Principal Developer, Hematopathology – Special Coagulation Laboratory, Department of Laboratory Medicine, Mayo Clinic, and Ian Burns, Senior Product Manager, Precision BioLogic.

