Stay Connected.
Get the inside scoop on company and product news. Subscribe to PBInsider, our quarterly e-newsletter, today!
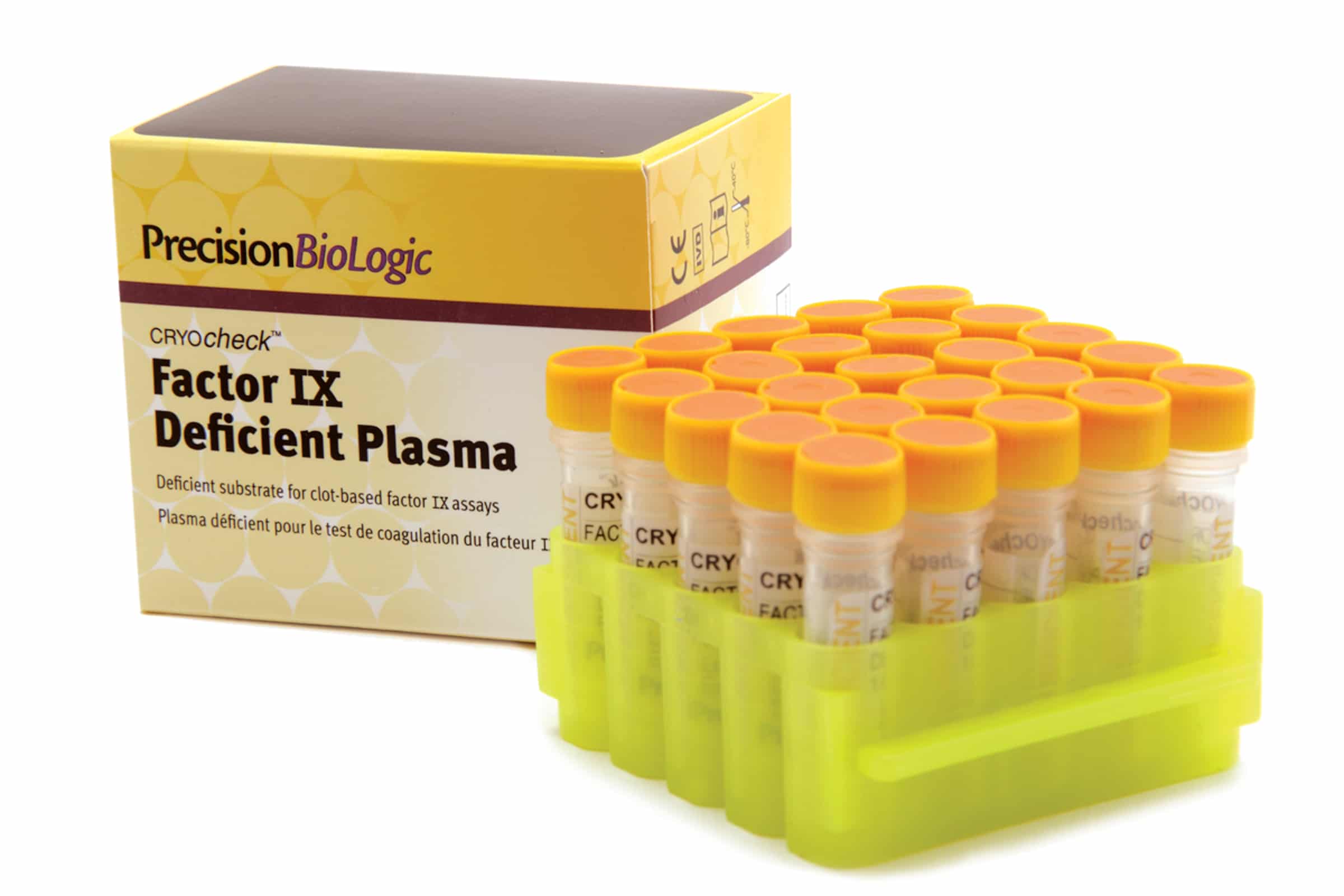
CRYOcheck™ Factor IX Deficient Plasma is immunodepleted, platelet-poor, and tested by both antigenic and functional assays to have less than 1% factor IX. Other coagulation factors have been assayed and results are provided on the Certificate of Analysis that accompanies each lot number.
between -40°C and -80°C
Frozen
3 years after manufacturing date
| Cat # | Format |
|---|---|
| FDP09-10 |
25 x 1.0 mL |
| FDP09-15 |
25 x 1.5 mL |